Nagi Bioscience leverages the SydLab™ One platform and its in vivo model to deliver precise, high-throughput toxicity assessments. Our solution accelerates safety and toxicology studies providing high translational value while eliminating the ethical concerns of vertebrate testing.
The fully automated, in vivo system evaluates multiple toxicity endpoints, including reproductive, developmental, neuro-, genomic, acute, and chronic toxicity, at costs comparable to cell-based assays.
By de-risking compound selection and streamlining toxicology workflows, we enable researchers to make informed decisions early in development, accelerating the path to safer pharmaceuticals, chemicals, and environmental agents.
C. elegans: A gateway to early toxicology
Caenorhabditis elegans is a cost-effective, ethically favorable model for toxicology studies with 60-80% of its genes homologous to humans. Its rapid life cycle and high-throughput capabilities enable efficient testing of chemicals, pharmaceuticals, and environmental pollutants.
The worm’s transparency allows real-time visualization of toxin effects, such as oxidative stress and neurodegeneration, providing insights into whole-organism responses. Genetic manipulability further supports the study of molecular toxicity pathways and biomarker identification.
Overall, C. elegans offers a streamlined, predictive platform for risk assessment, advancing safer compound development while reducing reliance on vertebrate models.
The Nagi Advantage
CUTTING-EDGE PLATFORM FOR HIGH-THOURGHPUT STUDIES
WORLD-CLASS EXPERTS DEDICATED TO YOU
POWERFUL IN VIVO MODEL FOR FAST AND ETHICAL TESTING
In vivo safety and toxicology studies at in vitro scale.
Our standardized protocols and automated workflow ensure consistent, actionable results.
In vivo model with high translational value without ethical concerns.
Very efficient treatment conditions.
C. elegans is a soil organism, providing high value to eco-toxicity applications.
Developmental toxicity, Reproductive toxicity, Acute and Chronic toxicity, Eco-toxicity, Neuro-toxicity.
From research to results: Our approach
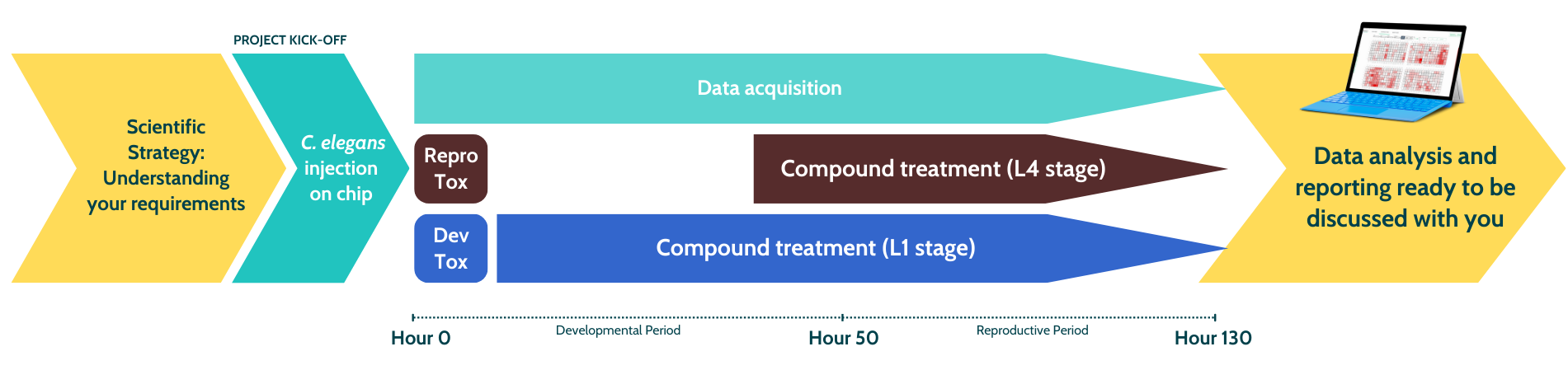
- Synchronized populations of C. elegans (L1 larval stage) are injected into the SydLab™ One platform (day 0).
- The SydLab™ One platform tightly controls the liquid environment to which each worm is exposed, including the treatments to be tested.
- For Developmental toxicity, worms are exposed to the test compounds from day 0, while for Repro-toxicity, worms are treated to test compounds after reaching sexual maturity.
- Images are acquired via time-lapse microscopy at the desired frequency.
- Our AI-based algorithm detects growth, reproductive and death parameters of individual worms, and provide a detailed analysis for the worm population.
- The experiment ends at the desired time (tipically after 5-7 days).
Readouts
- Growth dynamics and size
- Sexual maturity
- Egg accumulation
- Embryo survival
- Larvae emergence and accumulation
- Progeny size and growth
- NOAEL (No-Observed-Adverse-Effect Level)
Case Study: Reproductive toxicity assessment
Using Nagi Bioscience’s fully automated solution, we assessed the reproductive toxicity of the herbicide Paraquat and the cytotoxic chemotherapy drug 5-fluorouracil (5-FU).
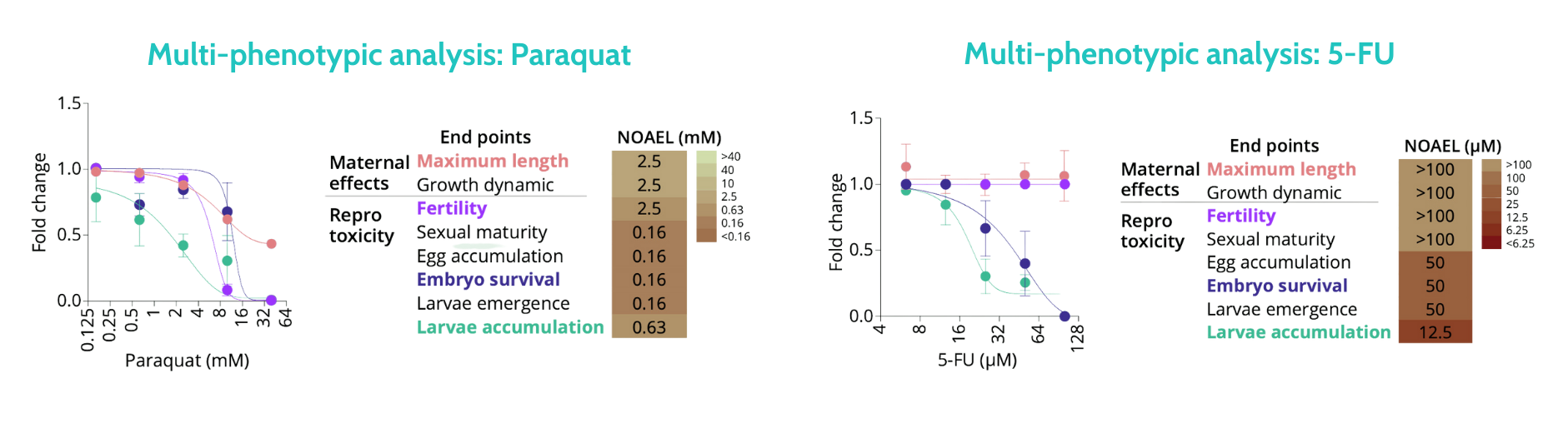
The SydLab™ One platform quantified the dose-dependent effects on maternal development (maximum length) and reproductive capacity (fertility, embryo survival, and larval accumulation). No-Observed-Adverse-Effect Level (NOAEL) were determined for both Paraquat and 5-FU at the end of the experiment.
In summary, Paraquat caused significant maternal adverse effects at high doses (NOAEL: 2.5 mM) and pronounced reproductive toxicity at lower doses (NOAEL: 0.16 mM). As expected, 5-FU exhibited no maternal adverse effects at the tested doses but demonstrated strong reproductive toxicity at intermediate doses (NOAEL: 50 µM).
Further references
Our commitment to you and your team
At Nagi Bioscience, we are more than just a service laboratory. We are a strategic partner dedicated to your success. Our team of experts brings 50+ years of experience, a passion for innovation, and a commitment to delivering actionable insights.
Initial Discussions:
Understanding your requirements
Send us your compounds of interest
Project kick-off:
Execute with Expertise
Report delivery:
High quality reports with actionable insights