A Tiny Worm with Transformative Insights for Human Health
The Gut Microbiome: A Cornerstone of Human Health
The gut microbiome, a dynamic ecosystem of bacteria, fungi, and viruses, is a cornerstone of human health, governing far more than simple digestion. This complex microbial community plays a critical role in immune function, mental well-being, and overall physiological balance.
Among the diverse microbes inhabiting the gut, bacteria like Bifidobacteria and Firmicutes have emerged as key players in maintaining health [1]. When this delicate balance is disrupted the consequences can be profound. Imbalances between different bacterial populations can trigger cascading health issues, including inflammatory bowel disease (IBD), obesity, and even neurodegenerative disorders [2].
Maintaining microbial equilibrium is no small feat with the immune system — both innate and adaptive — working tirelessly to preserve this diversity. Yet, factors like aging, poor diet, or antibiotics can skew the microbiome toward harmful species, leading to chronic inflammation, metabolic dysfunction, and conditions like leaky gut syndrome.
Why C. elegans? A Powerful Window into Microbial Interactions
Caenorhabditis elegans, as a model, is continuously bridging the gap in understanding the role of genetics, environment, and diet on the host physiology due to its simplified yet highly controllable microbial ecosystem.
What makes this tiny worm so extraordinary?
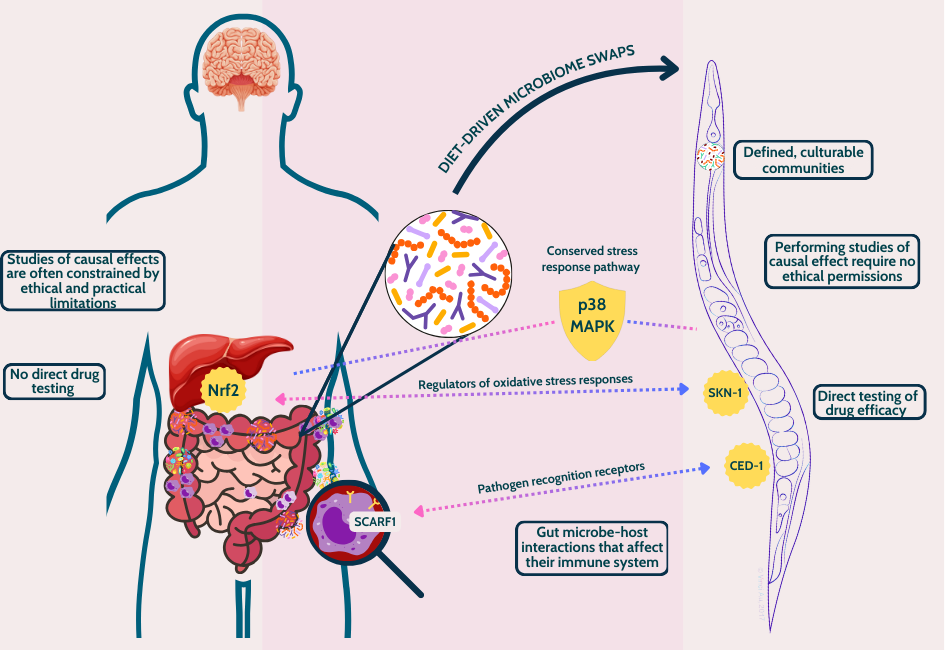
This nematode’s amenability to genetic engineering and transparent body allows investigation and real-time visualization of host-microbe interactions, respectively. Additionally, its short lifecycle and ease of cultivation under gnotobiotic (microbe-defined) conditions enable rapid, high-throughput experiments.
Furthermore, C. elegans also exhibits remarkable conservation with human biological systems.
- It shares a functional homolog with the human Nrf2 protein (SKN-1), which is crucial in maintaining cellular homeostasis and regulating responses to oxidative stress [3].
- Beyond its natural habitat, C. elegans has proven invaluable for studying human-relevant pathogens like Salmonella and Shigella decoding conserved defence mechanisms as p38 MAP kinase signaling cascade through monoculture studies [4].
- Conserved molecular players like CED-1 (akin to human SCARF1, a receptor for pathogen recognition), highlight the potential of this nematode as a model for studying immune-related pathologies and screening therapeutic interventions [5].
Finally, C. elegans bacterivore behavior make it an ideal model for studying complex microbiomes. By simply changing its diet, researchers can effortlessly perform microbiome transplantations [6].
Together, all these features make C. elegans a powerful tool for unraveling mechanisms of dysbiosis, microbial metabolism, and therapeutic interventions.
Bridging Microscopic Observations to Human Health Insights
The potential of C. elegans extends far beyond simple observation. Researchers have leveraged its unique characteristics to explore complex biological interactions that have direct implications for human health.
Host-Microbiome Interactions
Studies have revealed that the nematode’s native microbiome, though simple, mirrors functional interactions seen in humans. This enables precise studies of dysbiosis and immune responses. Dirksen et al. (2020) demonstrated how C. elegans can provide insights into host-microbe crosstalk, with immune responses to pathogens like Pseudomonas and Enterococcus closely resembling human gut defences [6].
Gut-Brain Axis & Neurodegeneration
Another hot topic discussed at recent microbiome and nutraceutical conferences is the emerging insights into the gut-brain axis. Recent studies have used C. elegans to explore neurological mechanisms that were previously difficult to investigate. Ma et al. (2021) showed how alpha-synuclein aggregation in C. elegans’ gut disrupts serotonin signaling, providing a model for understanding Parkinson’s disease (PD) origins. Complementing this finding, Nair et al. (2024) revealed that gut-derived serotonin exacerbates PD pathology, as an excess of gut-derived serotonin directly damages dopaminergic neurons, mimicking PD motor symptoms.
Drug Discovery & Therapeutic Screening
The potential of C. elegans for drug discovery represents a paradigm shift in pharmaceutical research. Unlike traditional target-based screening, which focuses on specific protein interactions, this approach leverages whole-organism models to identify compounds that can rescue or improve disease phenotypes.
The 2023 study by Thiruppathi et al. exemplifies this approach, demonstrating C. elegans’ unique capabilities in rapid probiotic screening. By identifying two lactic acid bacteria – Levilactobacillus brevis and Weizmannia coagulants– researchers discovered probiotics that:
- Enhance intestinal integrity
- Reduce oxidative stress
- Extend lifespan via conserved pathways (e.g., insulin/IGF-1, p38 MAPK).
This study underscores how C. elegans enables large-scale screening of microbial therapeutics to target age-related decline, with results translatable to mammalian systems.
Another groundbreaking study published in Cell Reports (Goya et al., 2019), further illustrated C. elegans potential in biomedical research by screening thousands of compounds to understand mitochondrial stress responses. The research revealed how mitochondrial dysfunction in the gut can trigger systemic aging — a pathway conserved in humans — and identified compounds capable of rescuing mitochondrial health.
Looking Towards the Future
The tiny C. elegans nematode is proving to be a giant in microbiome research, offering a transparent, genetically tractable model that helps us unravel the complex relationships between microbes and their hosts.
References
[1] Stojanov, S., Berlec, A., & Štrukelj, B. (2020). The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms, 8(11), 1715.
[2] Kennedy, P. J., Cryan, J. F., Dinan, T. G., & Clarke, G. (2014). Irritable bowel syndrome: a microbiome-gut-brain axis disorder?. World journal of gastroenterology: WJG, 20(39), 14105.
[3] Blackwell, T. K., Steinbaugh, M. J., Hourihan, J. M., Ewald, C. Y., & Isik, M. (2015). SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radical Biology and Medicine, 88, 290-301.
[4] Walker, A. C., Bhargava, R., Vaziriyan-Sani, A. S., Pourciau, C., Donahue, E. T., Dove, A. S., … & Czyż, D. M. (2021). Colonization of the Caenorhabditis elegans gut with human enteric bacterial pathogens leads to proteostasis disruption that is rescued by butyrate. PLoS pathogens, 17(5), e1009510.
[5] Lukácsi, S., Farkas, Z., Saskői, É., Bajtay, Z., & Takács-Vellai, K. (2021). Conserved and Distinct Elements of Phagocytosis in Human and C. elegans. International Journal of Molecular Sciences, 22(16), 8934.
[6] Møller, K. V., Wesseltoft, J. B., Malazarte, R., Kousgaard, S. J., Nielsen, H. L., Yashiro, E., & Olsen, A. (2023). Usage of cultured human fecal microbiota for colonization of Caenorhabditis elegans to study host–microbe interaction. Applied Microbiology, 3(4), 1130-1143.
[7] Dirksen, P., Marsh, S. A., Braker, I., Heitland, N., Wagner, S., Nakad, R., … & Schulenburg, H. (2016). The native microbiome of the nematode Caenorhabditis elegans: gateway to a new host-microbiome model. BMC biology, 14, 1-16.
[8] Ma, J., Wang, R., Chen, T., Jiang, S., & Xu, A. (2021). Protective effects of baicalin in a Caenorhabditis elegans model of Parkinson’s disease. Toxicology Research, 10(3), 409-417.
[9] Nair, T., Weathers, B. A., Stuhr, N. L., Nhan, J. D., & Curran, S. P. (2024). Serotonin deficiency from constitutive SKN-1 activation drives pathogen apathy. Nature Communications, 15(1), 8129.
[10] Thiruppathi, G., Mohankumar, A., Kalaiselvi, D., Velumani, M., Saravana Bhavan, P., Premasudha, P., … & Sundararaj, P. (2024). Geroprotective Effect of Levilactobacillus brevis and Weizmannia coagulans in Caenorhabditis elegans. Probiotics and Antimicrobial Proteins, 16(2), 589-605.
[11] Goya, M. E., Xue, F., Sampedro-Torres-Quevedo, C., Arnaouteli, S., Riquelme-Dominguez, L., Romanowski, A., … & Doitsidou, M. (2020). Probiotic Bacillus subtilis protects against α-synuclein aggregation in C. elegans. Cell reports, 30(2), 367-380.
Accelerate your research with Nagi Bioscience today
Ready to further enhance your formulations and substantiate health claims? Discover how our C. elegans-based platform can accelerate your pipeline while reducing costs and accelerating your path to market.