
Pre-clinical Testing
Bridging the gap between cell models and vertebrates
enbling in vivo testing at the in vitro scale
Bringing your drugs, chemicals and other compounds to the market is not only resource-intensive, but also takes an average of 10 to 15 years from discovery to market. Get full organism data while using the pioneer in vitro method Organism-on-Chip for testing your candidates during the pre-clinical phase.
enabling in vitro testing. Providing full organism data.

Bridging the gap with micro-organism models
The model organism C. elegans is a valuable alternative to rodent and cellular models in pharmaceutical research and toxicology for its strong relevance and proven translatability to human biology.
OBTAIN HIGHER REPRODUCIBILITY & STARDANDIZATION

no more manual protocols
While C. elegans are successfully used for phenotype-based drug screenings, identification of drug targets and mechanisms of action, there is a lack of protocol automation and standardization.
NOT ANYMORE WITH SYDLAB™ ONE

Boost your pipeline and lead optimization
Our laboratory solutions relying on the patented Organism-on-Chip technology unlock the unexploited potential of C. elegans for drugs and other substances screenings.
pre-clinical testing assays
MOLECULAR NEURODEGENeRATION ASSAY
Study of worm disease models
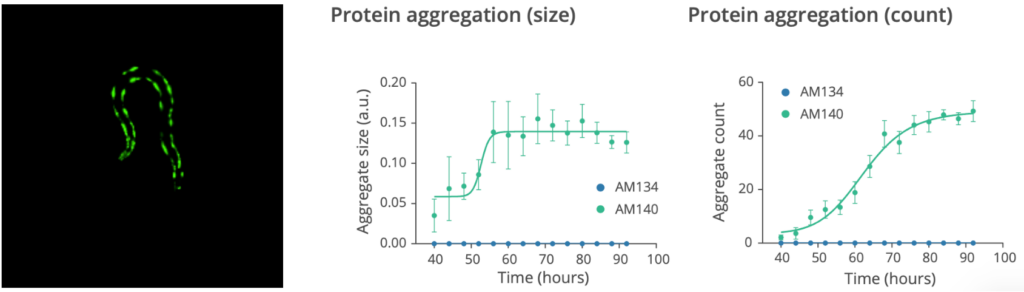
The assay to monitor the effects of test compounds on aggregate formation and aggregation dynamics in numerous C. elegans models of neurodegenerative diseases.
- Worms model of Amyotrophic Lateral Sclerosis (ALS)
- In vivo monitoring of the hSOD1 aggregates
- Quantification of the size and number of the aggregates during aging
METHOD DESCRIPTION
- For this assay we take advantage of the existing C. elegans GFP-reporter strains, modelling a variety of neurodegenerative disorders, such as Alzheimer’s, Parkinson’s, Huntington’s disease and amyotrophic lateral sclerosis (ALS), as well as the capacity of SydLab™ One to perform fluorescent imaging.
- A synchronized population of C. elegans is automatically injected into the Nagi™ Chips thanks to SydLab™ One at the first larval stage (L1). Worms are then confined within dedicated microfluidic chambers of the Nagi™ Chips and are continuously fed with an E. coli solution.
- Worms are chronically exposed to the test compounds starting from the last larval stage prior to sexual maturity (L4) for 85 hours (day 3 of adulthood). The entire process of culture and treatment administration is handled by SydLab™ One. The compounds to be tested are mixed with the E. coli solution fed to the worms. Freeze-dried OP50 E. coli are used as a food source for the whole duration of the experiment, preventing the metabolization of the tested molecules by the bacteria.
- The images of each microfluidic chamber are recorded every hour by SydLab™ One. Starting from 70 hours post worm injection (day 1 of adulthood), both brightfield and fluorescent images start to be recorded in order to monitor worm growth and protein aggregation dynamics in parallel. Time-resolved phenotypic readouts are then extracted from the collected images in both brightfield and fluorescent modes.
Check the data
MOTILITY PHENOTYPES DETECTION
NAGI MOTILITY PHENOTYPES DETeCTION
The assay is designed to evaluate the effects of test compounds on C. elegans behavior and motility, as well as to provide indications about neurotoxicity, muscular toxicity or premature aging process.
MOTILITY AND BEHAVIOR
NEUROTOXICITY assessment
MUSCULAR TOXICITY
Premature Aging
METHOD DESCRIPTION
- A synchronized population of C. elegans is automatically injected into the Nagi™ Chips by the SydLab™ One platform at the first larval stage (L1). Worms are then automatically confined within the dedicated microfluidic chambers inside the Nagi™ Chips and are continuously fed with an E. coli solution (no human intervention needed).
- The platform will chronically exposed the worms to the test compounds starting from the last larval stage prior to sexual maturity (L4) for 85 hours (day 3 of adulthood). The compounds to be tested are mixed with the E. coli solution. Freeze-dried OP50 E. coli are used as a food source for the whole duration of the experiment, since it prevents the metabolization of the tested molecules by the bacteria.
- Starting from 70 hours post-worm injection (day 1 of adulthood), SydLab™ One will record short video sequences ever 6 hours. Time-resolved readouts are then extracted from the acquired videos for a a diversified characterization of the worms’ motility and behavior thanks to the SydLab™ Analyzer software suite.
READOUTS
- Bending frequency
- Velocity
- Amplitude of the movement of the head
- Amplitude of the movementat the middle of the body
- Amplitude of the movement of the tail
- Curvature
Check the data
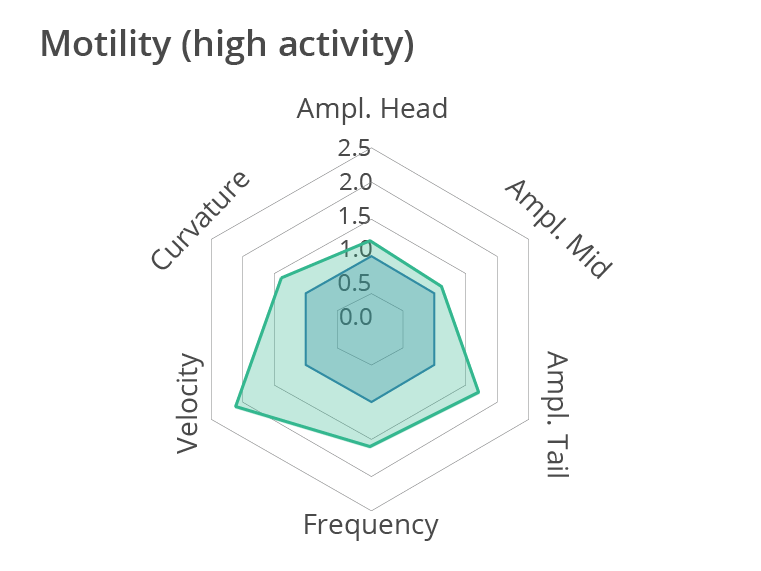
Representative multi-phenotypic fingerprint plot comparing a control worm and a more active worm at day 1 of adulthood, which includes readouts of body bends frequency, velocity, curvature as well as amplitude of the movement at the head, tail and middle of the body. In conclusion, this high-content motion analysis reveals very subtle changes in C. elegans movements, beyond average motility measurements. Data obtained by SydLab™ One.
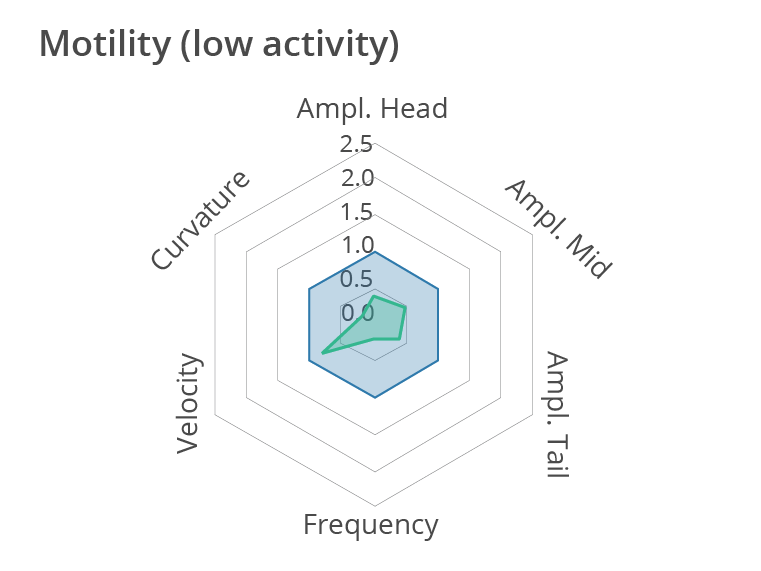
Representative multi-phenotypic fingerprint plot comparing a control worm with a less active worm at day 1 of adulthood, which includes readouts of body bends frequency, velocity, curvature as well as amplitude of the movement at the head, tail and middle of the body. In this case, this high-content motion analysis reveals very subtle changes in C. elegans movements, beyond average motility measurements. Data obtained by SydLab™ One.
UNIQUE RESEARCH NEEDS VERSATILITY
COMBINE THE MOTILITY FEATURES OF SYDLABtm One WITH OTHER ASSAYS
Customized pre-clinical Assays
Tailor-made bioassays on micro-organisms
Schedule a consultation with our experts to create a custom-made assay tailored to the specific needs of your research. At Nagi Service Solutions, we are with you at every step of the way, from experimentation design until the reporting of the results.


