
Biological models
BRIDGING THE GAP BETWEEN CELLS AND VERTEBRates
Powerful models for powerful datapoints
CAENORHABDITIS ELEGANS
Nature’s gift to science
Meet the powerful model organism
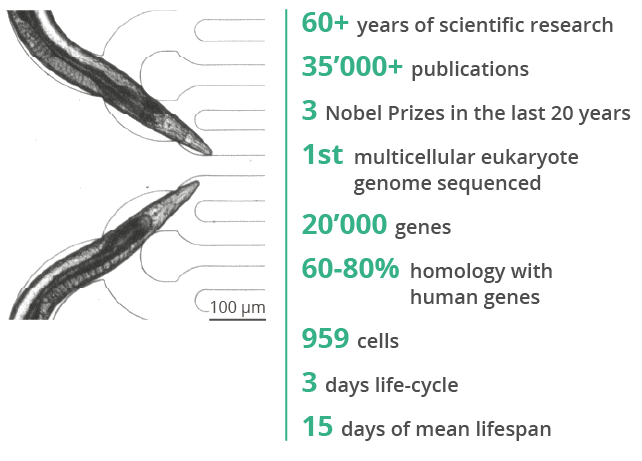
Caenorhabditis elegans has been known for more than 60 years as an important exploratory biological model in fundamental research. It helps in addressing various biological questions in the fields of aging, neurobiology, genomics, and developmental and cellular biology.
Models. in plural.
A world of possibilities opening up
To this date, large amounts of mutant, transgenic and “humanized” worm strains are available. More specifically, C. elegans is distinguished by its ease of genetic manipulations: single genes can be simply inactivated by feeding the worms with bacteria expressing dsRNA against the gene of interest (RNA interference method). Nowadays, any specific strain can be generated easily “on-demand” using modern CRISPR-Cas9 techniques.





strong relevance & proven translatability to human biology

C. elegans is a well-studied model with useful features for rapid investigations.
Keen to know more? Read the Nagi Blog!

Many pathways important in development, reproduction and modes of toxic action are conserved.

Concordant response areas include growth, development, LD50 ranking and neurotoxicity.
“If we understand the worm, we understand life.”
Sir John Edward Sulston, PhD. 2002 Nobel Prize of Medicine or Physiology
Small. Short life-cycle. not resource-intensive. powerful genetic toolkit.
Data is worth a thousand words
From drug development to toxicology screenings, find out all the areas of application of SydLab™ One.
OUR partners’ word
-
Failure in later stages of the R&D pipeline is especially costly. We actively pursue technologies to identify adverse toxicology as early as possible, such as Nagi Bioscience’s technologies.

Early Toxicology Safety – Agrochemical Company

Unfavorable toxicological profiles are a major cause of late failure in our discovery pipelines. Failure in later stages of the R&D pipeline is especially costly. We are actively pursuing technologies to identify adverse toxicology as early as possible, including through the use of model systems such as those being investigated by Nagi Bioscience.

Early Toxicology Safety – Agrochemical Company

-
Methodologies to assess lifespan and healthspan measures in a high-throughput manner are currently inadequate in the C. elegans field. The integration of Nagi’s technology opened many possibilities for us as a company.

CEO – Aging Biotech Company

Before proceeding to clinical trials, the efficacy of our existing drugs or combinations thereof for their positive impact on healthspan will be tested in the nematode C.elegans. However, methodologies to assess lifespan and healthspan measures in a high-throughput manner are currently inadequate in the C. elegans field. The integration of Nagi’s technology into Rejuvenate opened many possibilities for us as a company.

CEO – Aging Biotech Company

Unlock the full potential of small organisms with the all-in-one solution Sydlab One
Powerful biological models. powerful technology
First end-to-end automated system for small model organisms testing

Easy to integrate in your lab.

Tailored to your research.
Resources
-
Case Study
Automation of aging studies using Caenorhabditis elegans on the SydLab System
-
Publication
A method to identify and validate mitochondrial modulators using mammalian cells and the worm C. elegans.
Journal: Scientific Reports
-
Publication
An in vivo microfluidic study of bacterial transit in C. elegans nematodes.
Journal: Lab on a Chip
-
Poster
Automated high-content phenotyping of the nematode Caenorhabditis elegans: application to the toxicity assessment of perovskites.





