Do you remember a blog post from last year with the tagline “the change is here”? Maybe not, but we wouldn’t be doing our job well if we didn’t remind you about it. And why this throwback now? Because the FDA Modernization Act is officially approved!
Let’s recap a bit. The FDA Modernization Act was a bill proposal to start the transition towards non-animal testing methods for any type of substance/drug/product approval. As an example, for a drug to be eligible to go to clinical trials in the USA, it had to pass several tests performed on animals to ensure its alleged safety and efficacy on humans.
In this blog post from June 2022, we covered the main aspects of this bill when the US House of Representatives passed it. Later on, in September, the Senate gave more hope to the Act supporters by passing the bill too. And this leads us until today, where the FDA Modernization Act 2.0 is not just a long shot anymore.
The US President Joe Biden signed the Act just before entering into 2023, making the transition to alternative testing techniques and human-relevant methods official.
Science article here.
“Transition” sounds like PR jargon. What does the bill imply? For real
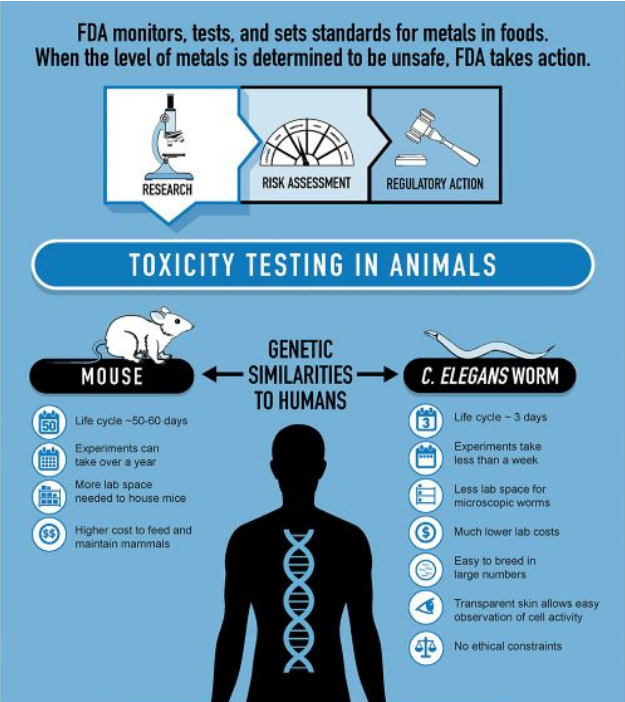
The headline is clear. The FDA no longer requires animal tests to go to the clinical trial phase. The Food and Drug Administration (FDA) is the US federal agency linked to the Health and Human Services Department in charge of guaranteeing and promoting public health through the control of any substance available in the market.
A definition is always cool, but let’s see what does this include:
It’s a long list as you can observe, and this means plenty of tests on animals as an indispensable requirement for approval.
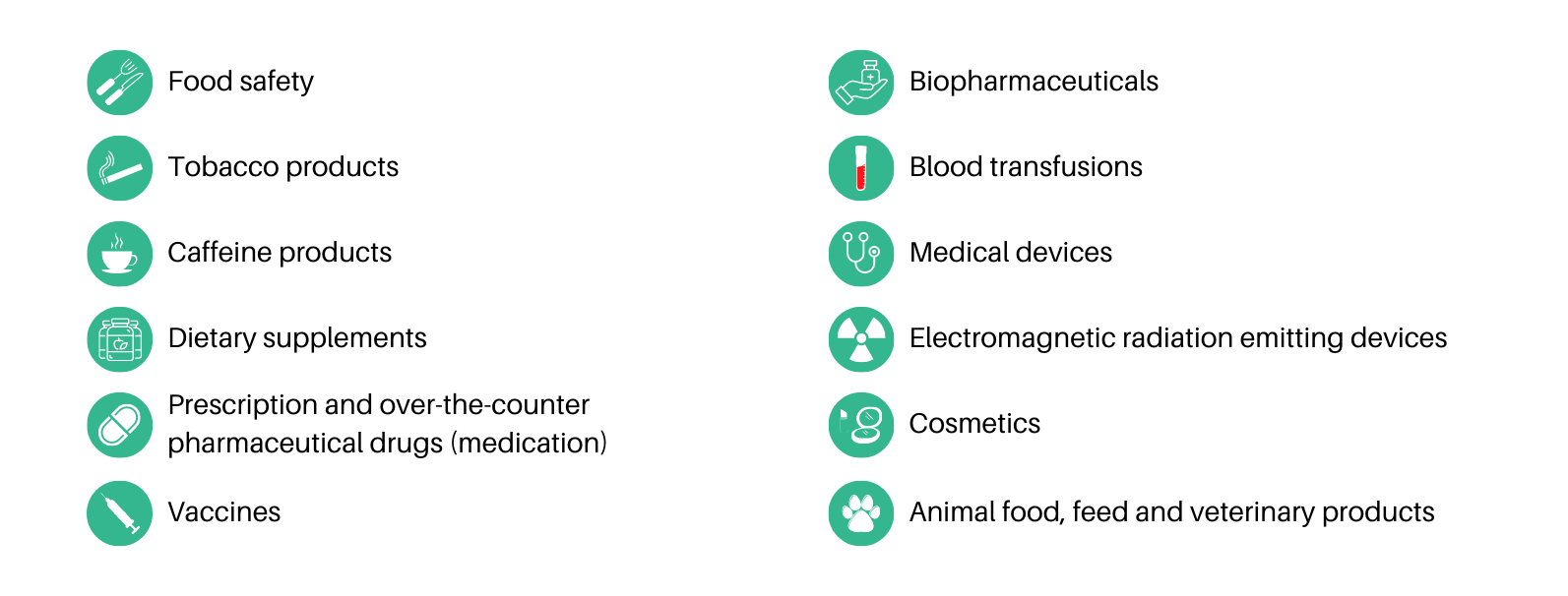
Problem? Well, we should say issues in plural. Here you have some of them:

First things first. It is not exempt from ethical concerns. At all.

In most cases, it does not prevent a drug from failing in clinical trials. In fact, more than 9 out 10 drugs tested on animals, later failed on human clinical trials.

Animal testing is extremely expensive, rising drug development costs and, hence, slowing the drug discovery pipeline. This ultimately affects patients.

We don’t talk only about drugs, but also about a bunch of other substances requiring toxicity clearing that can be done using alternative testing methods. Think of the cosmetic industry for instance: animal usage can be prevented while still launching safe products to the market.
Crash course over. Let’s start talking about what brought us here today: the FDA Modernization Act. The change of this directive is the first shift in more than 80 years (1938), allowing the FDA, for the first time, to approve a drug/biologic for clinical trials with the use of data obtained from non-animal tests.
Hence, is it a real paradigm shift? Obviously it is not a day-to-night change, but the FDA already put in motion several initiatives to explore and approve a wide range of existing alternative testing methods, as well as allocated budget to sponsor research to develop new ones. Conclusion: it is not an animal testing ban bill but allows researchers and companies to use alternatives methods when feasible.
What are those famous “alternative methods”? A recap
From Labs-on-a-Chip to in silico models, the alternative testing methods landscape is wide and has been in development for more than 15 years already. The traditional groups composing said environment are in vitro, in silico and ex vivo methods. But innovation is the heart of this sector, so today we have even more alternatives inside what we call the New Approach Methodologies (NAMs) toolbox.
C. elegans model: a new horizon at the FDA
One of these new methods is actually a highly established biological model. For more than 60 years in fact. Yes, you read it right, biological model. We are talking about our beloved nematodes C. elegans, a non-vertebrate 1mm roundworm who is non-sentient but with whom we share 60-80% of homologous genes. The FDA is already exploring this well-known model for regulatory toxicology tests as it is a really well characterized organism, far less cost-intensive than traditional animal testing and exempt of ethical issues as it is part of the NAMs toolkit.
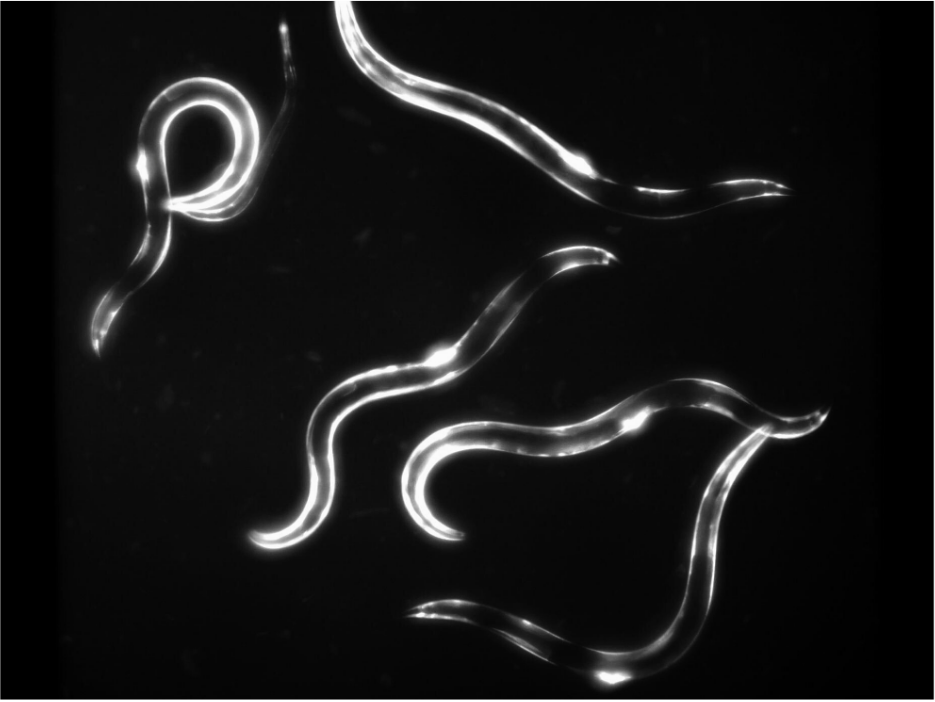
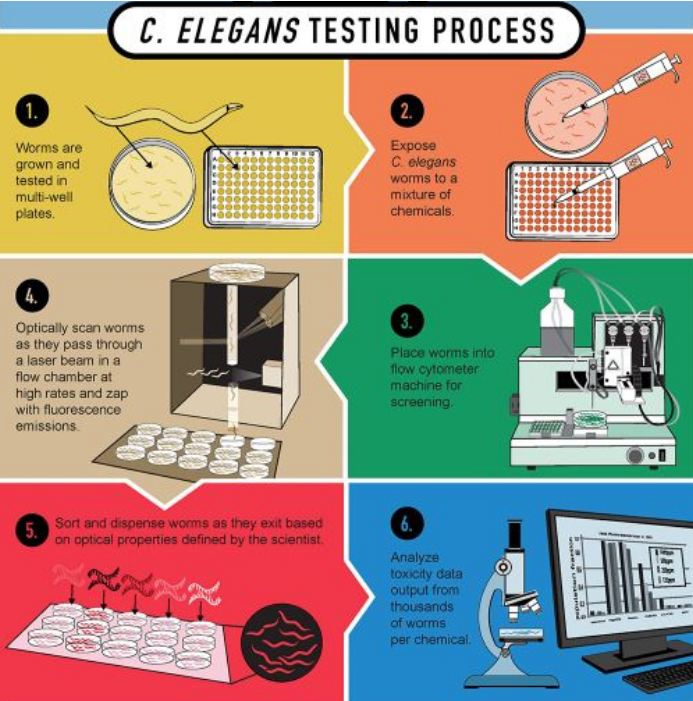
How does it work toxicity testing on C. elegans?
Well, this problem is not an issue anymore if we are being honest. See it with you own eyes

A trend becoming a wave
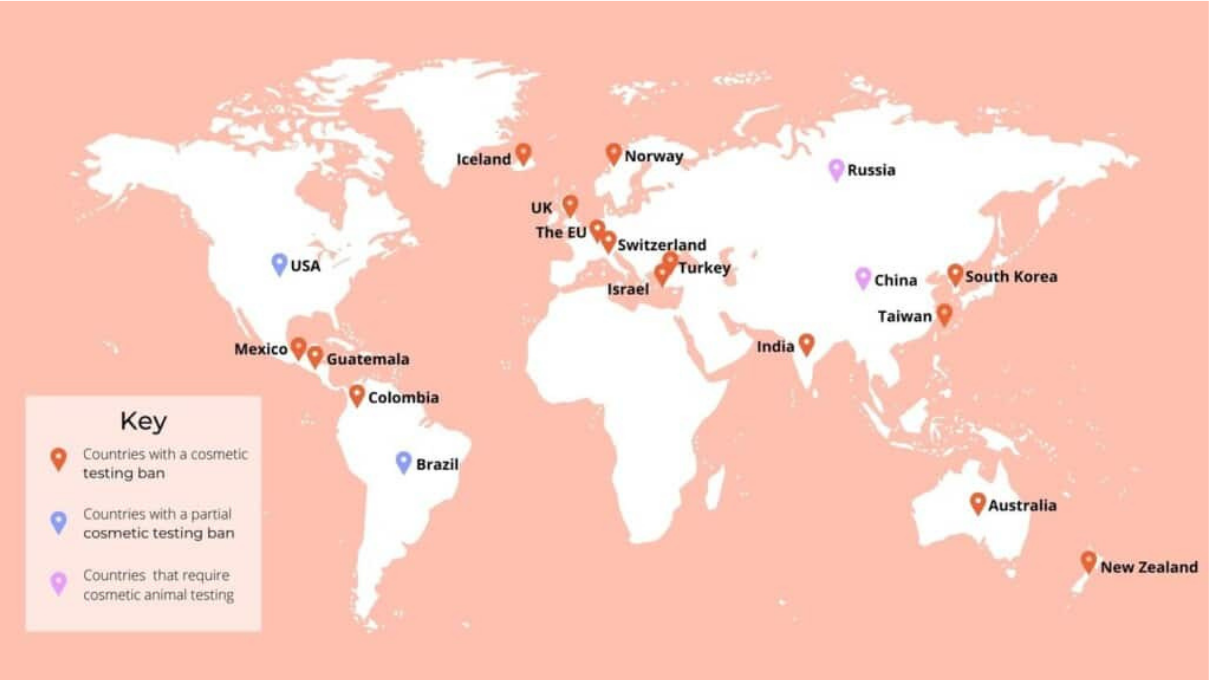
We have seen how many countries already banned animal tests for cosmetic products assessment. Actually, a total of 42 worldwide up to date. But this number is currently increasing. Continuing with the US, the state of New York just banned in December the use of animals for cosmetics’ testing, becoming the 10th state to do so. In Brazil, 12 out of the 26 states already took action and completely banned those tests also for cosmetic products.
While we still need the use of animals for certain safety and efficacy tests, as well as for research, the ban of those obsolete methods for testing substances is becoming more and more common. And why is that? Take cosmetics for example, reliable data can be obtained using alternative testing methods and still be safe for humans. What about drug development pipelines? While animal testing is still needed, the incorporation of alternative and human-relevant tests can help to rule out candidates that will fail in the next phases, avoiding unnecessary animal tests and creating faster and cost-effective pipelines.