The change is here. The US House of Representatives passed the FDA Modernization Act to transform the drug approval process and move towards a non-animal testing future

This week started with great news coming from US: the House of Representatives passed the FDA Modernization Act, which includes the drug approval process reform promoted by both Democrats and Republicans to transition towards non-animal testing methods in the next years.
Wayne Pacelle, president of Animal Wellness Action said: ‘Leaders of the House and lawmakers from both parties, recognize that the United States must lift archaic animal-testing mandates for drug development and replace that strategy with 21st-century methods grounded on human biology’.
95% of drugs fail during clinical trials in humans
Dated from 1938, the Federal Food, Drug and Cosmetics Act (FFDCA) strongly relies on animal testing for safety and efficacy assessment of substances, chemicals and drugs, which has been holding back R&D pipelines since then, according to the data presented by the evaluation commission. 95% of the drugs candidates selected based on current animal tests fail in human clinical trials, contributing more and more to increase costs and delays of drug development, which are estimated today to be between $1 and $6 billion and 10-to-15 years per drug respectively.
Besides the evident decrease of the industry efficiency over the last years, this lack of new drug discoveries affects the health and quality of life of millions of people.
Drawbacks of animal testing methods


How is the regulation in the rest of the world?
This is the current situation in the United States but, how is it in the other countries around the world?
For instance, the European Union states that animal models can only be used for experimentation if the expected benefits are greater than the risks, which is primarily measured in terms of animal pain, and when the objectives cannot be achieved using alternative methods.
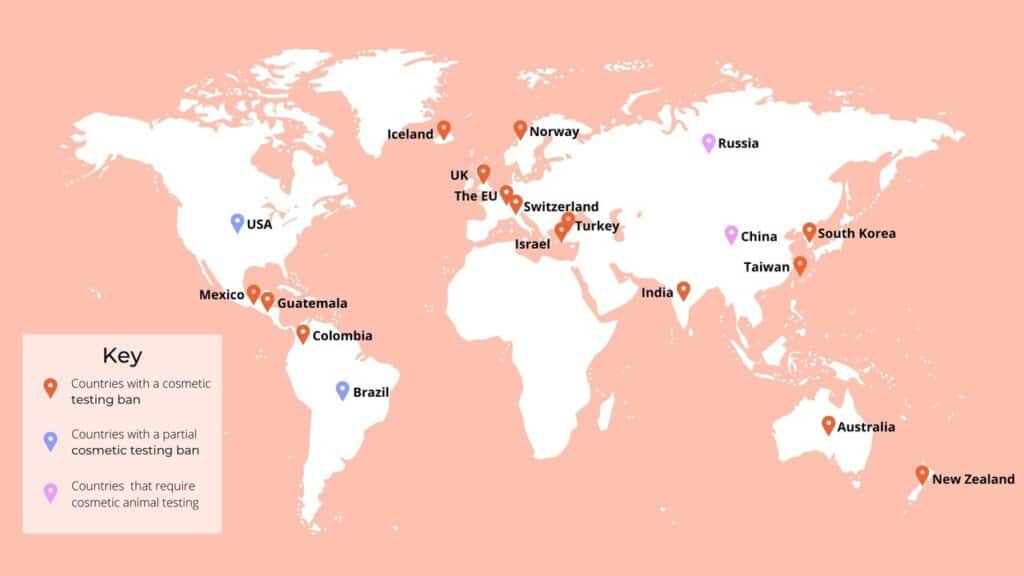
In the particular case of cosmetic products, however, a full ban came into force in the EU in 2013. Worldwide, 41 countries have enforced to limit or completely ban animal testing in the cosmetic industry, including the European Union territories, Australia, Colombia, Guatemala, Iceland, India, Israel, Mexico, New Zealand, Norway, South Korea, Switzerland, Taiwan, Turkey, the United Kingdom and several states in Brazil. Besides the ban in animal testing regarding in-country production, imported cosmetic products have to comply also with those regulations.
Nagi Bioscience – in vivo testing at the in vitro scale
At Nagi Bioscience we are committed to develop new 3Rs technologies heading towards a new biological testing future: scalable, efficient, ethical, sustainable and animal-free. And today we are proud to present you the solution: the first Organism-on-Chip technology.
Using Nagi’s Chips in our pioneer SydLab System, the automation of drugs and chemicals screening on the model C. elegans is now a reality. But, what are C. elegans? The nematode discovered in 1900 by Émile Maupas has been since then key in numerous scientific discoveries, including for instance the Insulin Growth Factor (IGF-1) signaling pathway or its contribution to the Human Genome Project. As part of the alternative testing methods toolkit, C. elegans can provide whole-organism data bridging the gap between in vitro methods and vertebrates.
One of the major drawbacks in the usage of alternative testing methods is the lack of standardization and automation, and the protocols for experiments using C. elegans are not an exception. With the aim of reducing research pipeline drawbacks and speeding-up R&D processes, our team developed the SydLab System: An all-in-one laboratory device combining state-of-the-art microfluidic technology, robotics and A.I. to enable, for the first time, fully automated high-content drug and chemical screening on C. elegans.
The future of alternative testing methods – A glimpse into the market
It is not surprising that the governmental and legislative support worldwide is fueling the alternative testing methodologies market. But, how are they shaping the market?
In 2021, the Business Research Company described the exponential adoption of New Approach Methodologies technologies (NAMs) for pre-clinical research due to the risk/benefit balance: high probability of pre-clinical trial failure and cost-effective assays. The same report claimed an expected CAGR of 9.08% for the global non-animal alternative testing market resulting in a total value of $1.78 billion for this current year 2022. Despite the traditional animal testing sector is still reporting a higher market share ($10.74 billion in 2019), its expected CAGR is estimated to be 4.27% during 2019-2025 and 2.46% between 2025 and 2035.
Hence, the companies’ aim to reduce R&D drawbacks and costs, the governmental financial support for the research of new technologies, and the increasing legislative acts in favor of non-animal methods are the main reasons driving NAMs to increasingly steal market share to traditional animal testing methods.
Interested in knowing more? Check out our other blog posts