The Challenge
Ensuring product safety isn’t optional, it’s the foundation of credibility, regulatory approval, and consumer trust.
Yet traditional toxicity testing is often time consuming, costly, and dependent on mammalian studies that raise ethical and practical limitations.
Early-stage in vitro models often fail to reproduce complex biological responses that become evident only in living organisms, leading to risks that emerge later in development.
Key trends
Regulatory agencies like REACH in the EU highlight the increased need for chemical testing, while DTSC reports inadequate toxicological studies for high-production volume chemicals.
FDA is actively promoting a new non-animal safety assessment framework, driving the adoption of New Approach Methodologies (NAMs).
There is a growing need for in vivo safety testing approaches that combine fast, reliable, and ethical standards.
Such methods enable early compound de-risking, reduce reliance on animal models, and provide reproducible data to support every stage development decisions.
Revolutionizing early-stage toxicity assessments with the Organism-on-Chip technology
Predictive In Vivo Insights
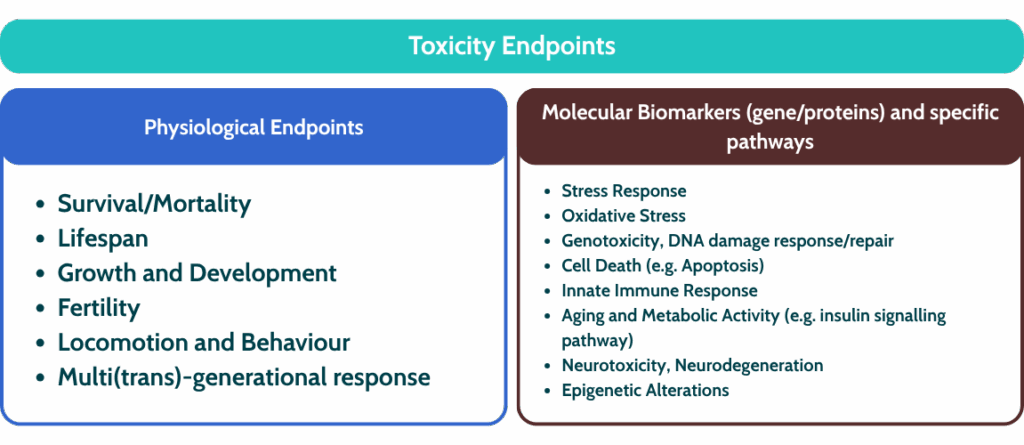
Beyond being an ideal NAMs model, C. elegans has proven to be a highly effective and versatile tool for early toxicity testing, with the capacity to assess a broad range of endpoints.
Its ease of genetic manipulation enables the study of gene expression changes in response to toxicants and providing mechanistic insights into gene regulation, biochemical pathways, and the effects of pollutants, chemicals, and nanomaterials.
Automation and Reproducibility
From experiment design to data analysis, we deliver actionable insights so you can advance fast and with confidence.
Our SydLab™ One platform provides end-to-end automation, reducing human error and ensuring consistent, high-quality data in DART and Eco-Tox assays. Introduce the Organism-on-Chip technology to support confident decision-making at every stage.
High-Throughput, Cost-Efficient Safety Testing
With the ability to screen hundreds of compounds per month, toxicologists can identify liabilities early, flag for possible late-stage failures, and accelerate timelines without compromising safety.
Our solutions for safety testing
Nagi™ DART
High-throughput in vivo Developmental and Reproductive Toxicity assay.
Multidimensional insights in just 5 days.
Eco-Toxicity testing
– A Custom assay
With Nagi Bioscience, you gain:
Early de-risking of compounds
Detect adverse effects in vivo within days instead of months.
Rapid turnaround
Safety-relevant results available in 5-7 days.
Regulatory relevance
Generate datasets aligned with NAMs frameworks and evolving global guidelines.
DART, Eco-Tox, Biological Age and Healthspan metrics
Quantify safety hazards and even subtle toxic effects on organismal vitality, not just survival.
Reduced costs and ethical impact
Lower dependency on vertebrate testing and build strong scientific evidence that strengthens submissions to regulatory authorities for starting vertebrate studies.
We help toxicologists in every industry ensure product safety — faster, with greater reliability, and with stronger ethical standards.

“We had a highly positive experience with Nagi’s services. Their innovative assays provided a reliable alternative to mouse studies by leveraging the short lifespan of C. elegans and the high conservation of our target from worms to human.“

“Their services revealed new insights and opened up exciting possibilities for exploring the potential of our strains in health applications related to healthy aging, helping us advance our research further.“

“Their high-throughput approach provided clear, quantifiable insights into the lifespan and functional effects of our lead combinations— critical data that helped de-risk and prioritize our therapeutic strategy.“









